A Conformational Switch in Bacteriophage P22 Portal Protein Primes Genome Injection
A Conformational Switch in Bacteriophage P22 Portal Protein Primes Genome Injection
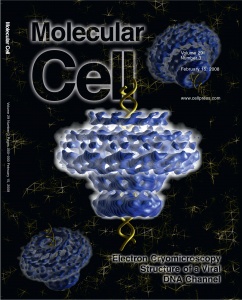
A Conformational Switch in Bacteriophage P22 Portal Protein Primes Genome Injection, Hongjin Zheng, Adam S. Olia, Melissa Gonen, Simeon Andrews, Gino Cingolani, and Tamir Gonen. Molecular Cell, 29, 376-383 (2008). Double-stranded DNA viruses such as herpesviruses and bacteriophages infect by delivering their genetic material into cells, a task mediated by a DNA channel called ‘portal protein.’ In the work described in this paper, electron cryomicroscopy was used to determine the structure of bacteriophage P22 portal protein in both the procapsid and mature capsid conformations. The authors found that, as the viral capsid undergoes major conformational changes during virus maturation, the portal protein switches its own conformation to erect a large DNA scaffolding domain directly above the pore of the DNA channel. It is possible that, in this way, portal protein primes DNA for injection and directly couples completion of virus morphogenesis to a new cycle of infection.
Hongjin Zheng, Adam S. Olia, Melissa Gonen, Simeon Andrews, Gino Cingolani, and Tamir Gonen – University of Washington